The article below on Locomotive Boilers is based on some of my notes from that period.
So you want to drive a steam locomotive?
It takes some time to really learn the job. In the pre-war days of the 'Big Four' (LMS, GWR, LNER, SR), you could be working on the footplate for 20 years or more before you graduated to become a driver. In that time, you were expected to have picked up a lot of knowledge, a lot of experience. The engine driver is like the captain of a ship: on the footplate, he is in charge. Whatever happens, it's up to the driver to decide what to do. The confidence to take the right actions in an emergency depends upon familiarity with the construction and working of engines. Well, let's make a start and keep it fairly simple.
Great Western large taper boiler (less smokebox)
Click for larger version.
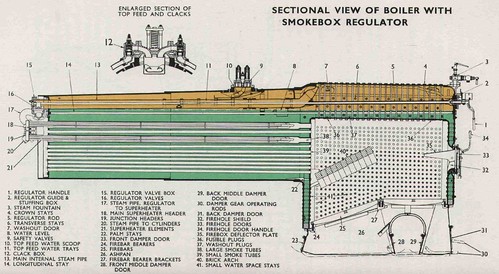
All the great locomotive designers have understood that the key to a successful engine design is its ability to boil water. If you can't make steam in the first place, everything else is a waste of time. Here, in the yard at Tyseley, we've a number of locomotives under restoration and this gives us the chance to look at a boiler which has been lifted out of the frames. This is a Great Western design boiler, taken off an 8-coupled freight locomotive, but fairly similar to the boilers used on all larger GWR engines (see diagram above). The principles are the same as almost all steam locomotives in the world - the classic 'Stephensonian' boiler going right back to the Rocket, built in 1829! Looking at the boiler, supported on wooden packing on the ground, it's apparent that the boiler unit is, in fact, three parts joined together. In the middle is the boiler itself, recognisable by its fairly cylindrical shape. This is chosen to give it sufficient strength to contain the high-pressure steam which will collect above the surface of the boiling water which will fill the boiler when it's in use. In a lot of locomotives the boiler is a proper cylinder and we call it a 'parallel' boiler. But this one is a bit more complicated, as it is coned slightly towards the front of the engine and is called a 'taper' boiler. The main reason for this tapering is to improve the internal convection currents in the water as it is boiled. The back end of the boiler is joined to a complex structure called the firebox, bristling with riveted stay heads. This is where the fuel is burnt to produce the heat energy which boils the water. The front end of the boiler is joined to a simpler, cylindrical tube called the smokebox which carries the chimney.
Let's have a closer look at the firebox. That's where the coal goes, so that's where it all starts. The firebox is rectangular in plan and towers above us in a sort of inverted-U section, open at the bottom. The front end of the firebox is joined onto the boiler barrel and the back end is solid, apart from a number of small piercings for mounting various boiler fittings and a circular hole about 18-inches diameter, called the firehole. That's where the coal goes, so the cab, sits immediately behind the firebox with the footplate, where the driver and fireman work, built up to a little below the firehole. This arrangement is supposed to make it easy for the fireman to swing round and collect coal from the bunker or tender containing coal behind the cab, then deftly deliver each shovelful through the firehole and onto the right spot on the fire. As you'll see when we get on the road, it's not always that easy.
This boiler is under restoration, so many of the stays are missing. Instead, you can see a tapped hole in the steel outer firebox which will receive the screwed stay which is riveted over after fitting to make it completely steam tight. Looking through the hole in the outer firebox, you can see an inner firebox wall about 3-inches away, with a corresponding threaded hole. The stays, thousands of them on a large boiler, suspend the inner firebox from the outer firebox making a strong, rigid structure. Because this boiler has been lifted up on sleepers, we can actually duck under the bottom of the firebox and stand up inside the firebox. Now the structure is a little clearer. We're standing in an upside-down box, open at the bottom. This inner firebox is made of copper, so that it can quickly conduct the heat from the fire, which would be where we're standing, through to the water on the other side, trapped in the space between inner and outer fireboxes. Around the base of the firebox, inner and outer fireboxes are riveted together through a substantial foundation ring. Brackets projecting inside the firebox near the foundation ring are used to carry a series of cast iron firebars which, when work on the stays is complete, will sit across the bottom to support the burning fuel. The firebars have ends wider than the middle so that narrow air spaces are left between adjacent firebars allowing combustion air to be drawn up through the burning fuel.
Perhaps we should briefly discuss the chemistry of the combustion of coal. Coal is a naturally-occurring substance extracted from the ground comprising mainly carbon and carbon in association with hydrogen. Analysis varies widely according to where the coal is found. Iron, sulphur and ash-forming impurities are normally present. The importance of coal is that, if warmed to a few hundred degrees Farenheit, it emits vapours which will burn in the presence of air. That means, principally, that the hydrogen in the coal combines with oxygen in the air to form water vapour and the carbon in the coal combines with oxygen in the air to form the colourless, odourless gas called carbon dioxide. These are the same combustion products given off by cars burning petrol or diesel which, like coal, are also hydrocarbons but in liquid form more readily burnt than coal. In all cases, the chemical reactions in which oxygen combines with hydrogen and carbon in the fuel gives off a lot of heat (the reactions are said to be 'exothermic') and this is what we're after - heat energy which can be converted into mechanical energy to make the engine go.
Coal doesn't burn that easily. We have to raise its temperature to about 800 degrees Farenheit (400 degrees Celsius) before it starts to emit a burnable gas. This is the oily, tarry volatile part of the coal, made up of complex hydrocarbons which give off heat when they are oxidised by burning in sufficient air. This air supply is critical. The oxygen needed for burning is only around 1/5 of air by volume. Air is mainly nitrogen, which is an inert gas playing no active part in our combustion. For the volatile hydrocarbons to burn away properly, we have to make sure that there's sufficient air to provide all the needed oxygen. A lot of the details of a locomotive boiler are concerned with arranging and controlling this air supply. If we don't provide enough oxygen, the hydrocarbons drift away as a yellowish gas and the heat we could have produced by burning them is lost.
When the volatiles have burned away, what remains is principally carbon, as coke. This will burn away fairly slowly. If sufficient combustion air is provided, then the carbon oxidises to carbon dioxide, giving off considerable heat in the process. This reaction takes place at a considerably higher temperature - around 2800 degrees Farenheit (about 1500 degrees Celsius). If the air supply is restricted, the carbon oxidises to carbon monoxide. This reaction takes place at a much lower temperature and liberates far less heat. Again, we lose energy.
So a conscientious fireman will understand the reactions taking place in the firebox and will regulate matters so that the coal is burnt efficiently, releasing the maximum amount of energy. That way, he will use less coal and use less physical energy himself.
As each individual lump of coal emits burnable vapours, it becomes smaller and smaller and sinks down towards the firebars, as fresh coal is added on the surface. Eventually, all the carbon is gone and what remains is the incombustible ash. If you're lucky, this will fall through the air spaces between the firebars and be collected in the ashpan suspended underneath the firebox. But many coals contain a certain amount of iron, often in the form of iron pyrite (Fool's Gold), comprising iron in association with sulphur. The sulphur in the iron pyrite is released as a gas, sulphur dioxide, discussed below, whilst the iron combines with the ash content to form a solid slag or clinker. This can spread out across the firebars as a solid sheet, blocking the air spaces and reducing the usable area of the firegrate. This problem is called 'clinkering' and may announce itself by the boiler suddenly stopping steaming as the grate chokes up. So a fireman will pay careful attention to the fire, firstly to minimise the production of clinker as much as possible and, where unavoidable, to break up the clinker before the pieces become too large.
The origin of the coal will determine its chemical analysis and thus its clinker-forming characteristics. Not all coals are suitable for locomotives. Coals with a high sulphur content are problematic. As with iron pyrite, the sulphur combines with oxygen in the combustion air to give sulphur dioxide which then further reacts with the water vapour produced by the combustion of the volatile hydrocarbons to give sulphuric acid - 'acid rain'. Not only is this environmentally unfriendly, but the smoke tubes in the boiler leading the gases away from the firebox to the chimney are damaged by the acid.
Unless very carefully controlled, burning coal can be a very dirty, smoky business. The volatile gases given off, if incompletely burned, give a dark grey exhaust at the chimney. In addition, small particles of coal tend to get carried away before they are burned, appearing as black smoke at the chimney.
The original Act of Parliament allowing steam locomotives to be used on railways (Railway Act 7th George IV) was very concerned about the tendency to produce black smoke and the legislation said that locomotives must "consume their own smoke". In 1829, when the design of the Stephensonian boiler evolved, the only way to achieve this with the readily-available fuels was by using coke, not coal to power the engine. As discussed earlier, coke is what you get when you heat coal and drive off the volatile hydrocarbons. This has to be done with a limited supply of air, allowing the hydrocarbons to be released, but preventing the second stage of burning as the carbon burns. Originally, this was done by digging a pit in the ground, filling it with coal, setting fire to it and covering the coal to restrict the available air. Although coke burning was effective as a means of preventing smoke, the separate production process was inconvenient and a number of locomotive engineers experimented to find a way of burning coal directly in the firebox. The answer, when it came, was relatively straightforward. Matthew William Kirtley of the North Midland Railway (later the Midland Railway) realised that gases and fine particles emitted from the burning fuel in a locomotive firebox were being drawn away from the hot area immediately above the firebed, where conditions were favourable to complete combustion, too quickly for all the reactions to take place. Some of the combustion air was being drawn straight through the firebed and through the smoke tubes to the chimney, without its oxygen being used up in helping to completely burn the fuel. What was needed was some means of promoting more intimate mixing of the combustion air with the hydrocarbon gases and small coal particles in the space above the burning fuel. The answer was an inclined arch set across the width of the firebox, made from heat-resisting refractory bricks. This deliberate obstruction to gases leaving the firebox via the smoke tubes causes the gases to be reflected back towards the fire and swirl around above the firebed, delaying their exit and giving an opportunity for all the oxygen in the air to be used up in complete combustion. When the engine is worked hard, the brick arch itself becomes very hot and this, in turn, helps to keep the gases at the high temperature needed for complete combustion.
There are two sources of combustion air to a locomotive fire. The first has been discussed: air drawn from under the locomotive, through the ashpan , through the slots in the firegrate and through the burning mass of fuel. This is called primary air, or 'bottom' air. But the firehole provides not only the means for getting coal into the firebox, but also a means of allowing further air into the firebox. This is called secondary, or 'top' air. Secondary air is introduced above the burning fuel, providing additional air which mixes with the gases being given off by the coal, supplying additional oxygen to allow complete, efficient combustion of the gases. This secondary source is used as required, the fireman judging when to run with the firedoors closed and when to run with the firedoors open or partially open. The fire will be hottest running on primary air alone, but unless there is adequate draught on the fire to pull sufficient air through the firebed, there is the chance of incomplete combustion or the production of excessive smoke. Opening the firedoors allows in additional air but since secondary air, unlike primary air, is not pre-heated by passing through the firebed, the effect will be to slightly cool the fire.
The main function of the ashpan, hung underneath the firebox, is to collect the ash and cinders produced by the burning coal as they drop through the spaces in the firegrate. A traditional ashpan is provided with a solid bottom and solid sides made from sheet steel but one or both ends are arranged with hinging doors called dampers. These can be manually opened and closed by a mechanical linkage to the footplate, allowing the fireman to manually regulate the amount of primary air. Gravity is normally arranged to keep the dampers closed and there is frequently a series of notches in the damper control lever allowing the fireman to latch the damper door at different openings.
During disposal of the locomotive on shed, all the dampers are opened wide allowing the disposal staff to stand in a pit and rake out all the ash. This is essential as, once the ashpan is full of ash, it prevents combustion air from passing through the ashpan to reach the fire. There are many different layouts of ashpans, dampers and damper control linkages. On small locomotives, there may only be a back damper with the front of the ashpan solid. Two dampers are most common, one front, one back (sometimes termed 'fore' and 'hind'). In some locomotive designs, the trailing axle gets in the way of the ashpan, which may have to hump up to clear the axle. This may require additional damper doors to ensure that all the ash can be cleared. In large GWR locomotives like 'Castles' or 'Kings', two completely separate ashpans are provided, one in front of the trailing axle and one behind. These ashpans are provided with a total of four dampers, two front and two back. 'Merchant Navy' and 'West Country' Pacifics have a screw arrangement operated from a handwheel in the cab to set the position of the dampers.
Clinker is the enemy of the fireman. The various overgrown pokers carried on engines, called fireirons, are provided to let the fireman poke about in the fire, to break up clinker and assist ash in dropping through the firebars into the ashpan. There's usually a selection to reach different parts of the firebox. In a narrow firebox of a large engine, the fireiron is about twelve feet long, so it's no easy task to wrestle it down from the tender, rake it through the mass of fire and then, with the working end now red hot, lift it back onto the tender. 'Cleaning' the fire is best done whilst stationary but, if the fireman is caught unawares by an engine that won't steam, the process has to be done on the road. But with good coal fired intelligently onto a fire that was clean to start with, an engine can steam all day without attention from fireirons.
Well, there's lots more to say about boilers but that'll do for now.

